10600000
Total TB incidence (2022)
7465430
TB new and relapse cases (2022)
1300000
TB deaths (2022)
Tuberculosis - A Global Burden
Tuberculosis (TB) is an infectious disease caused by various strains of mycobacteria, usually Mycobacterium Tuberculosis, which is spread from person to person through the air when an infected person coughs, sneezes or otherwise transmits saliva through the air. While the disease typically attacks the lungs, it can also affect other parts of the body. However, not everyone infected with TB bacteria becomes sick. As a result, two TB-related conditions exist: latent TB infection and TB disease.
Persons with latent TB infection do not feel sick and do not have any symptoms. They are infected with M. tuberculosis, but do not have TB disease. Overall, without treatment, about 5 to 10% of infected persons will develop TB disease at some time in their lives. About half of those people who develop TB will do so within the first two years of infection. For persons whose immune systems are weak, especially those with HIV infection, the risk of developing TB disease is considerably higher than for persons with normal immune systems.
Diagnosis of Latent Tuberculosis Infection (LTBI)
There is no gold standard test for LTBI. Indeed, the low tissue bacterial burden associated with LTBI works against any diagnostic strategy focused on the identification of the bacteria or its components. The diagnosis of LTBI is rather indirect and relies on evidence of a cellular immune response to mycobacterial antigens. The most commonly used tests for LTBI diagnosis are the intradermal tuberculin test (TST) and IGRA. Persons who have received BCG (either as a vaccine or for cancer therapy) and persons from groups that historically have poor rates of return for TST reading, are preferred for IGRAs testing.
Wantai's TB-IGRA Solutions
Wantai’s TB-IGRAs (Interferon Gamma Release Assay) are intended for use as an aid in the diagnosis of TB infection including both latent Tuberculosis infection and Tuberculosis disease.
How does IGRA work?
TB-IGRA measures a person’s immune reactivity to Mycobacterium Tuberculosis. If a person is infected with M. Tuberculosis, his or her T-lymphocytes will release Interferon Gamma (IFN-γ) when mixed in-vitro with antigens derived from M. Tuberculosis. Wantai’s TB-IGRAs are quantitative or semi-quantitative tests for detection of IFN-γ released by the T-cells that have responded to in-vitro stimulation by Mycobacterium Tuberculosis antigens in human blood samples.
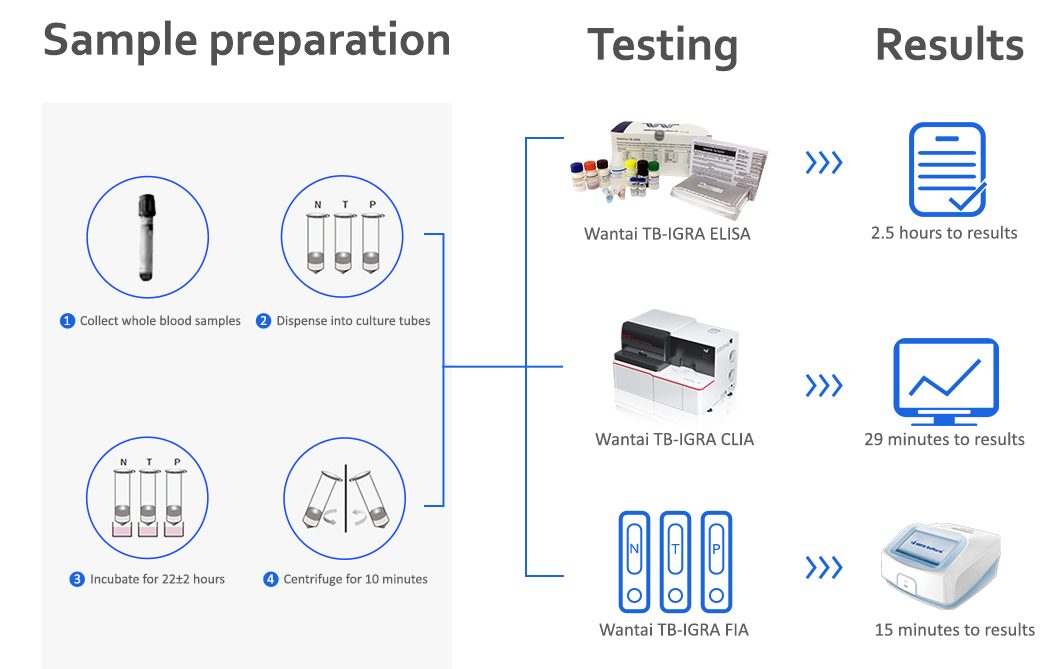
Wantai TB-IGRA is available as manual or semi-automated ELISA, fully automated CLIA or in Point-of-care format.
Wantai's TB-IGRA Workflow
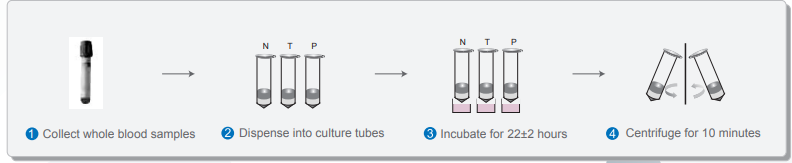
Single-tube blood collection:
1. Uses a single blood collection tube containing lithium heparin as the anticoagulant.
2. Ensures a simplified rapid blood collection process
3. Enhances patient satisfaction
4. Increased time flexibility at the blood draw site
Standard centrifugation and automatic process:
Standardized results optimal harmonization between urgent, specialty and routine tests.
Wantai CLIA immunoassay analyzers
1. Wantai CLIA immunoassay analyzers are designed for both specialty and routine tests to help your laboratory handle multiple patients and tests simultaneously with minimal user intervention.
2. Reduced turnaround time
3. Optimal cost management
Recommendations
Wantai TB-IGRA ELISA is included in the Stop TB Partnership Manual on selection and use of interferon-gamma release assays (IGRAs) for testing for TB infection and in the WHO's policy statement on the use of alternative interferon-gamma release assays for the diagnosis of TB infection.
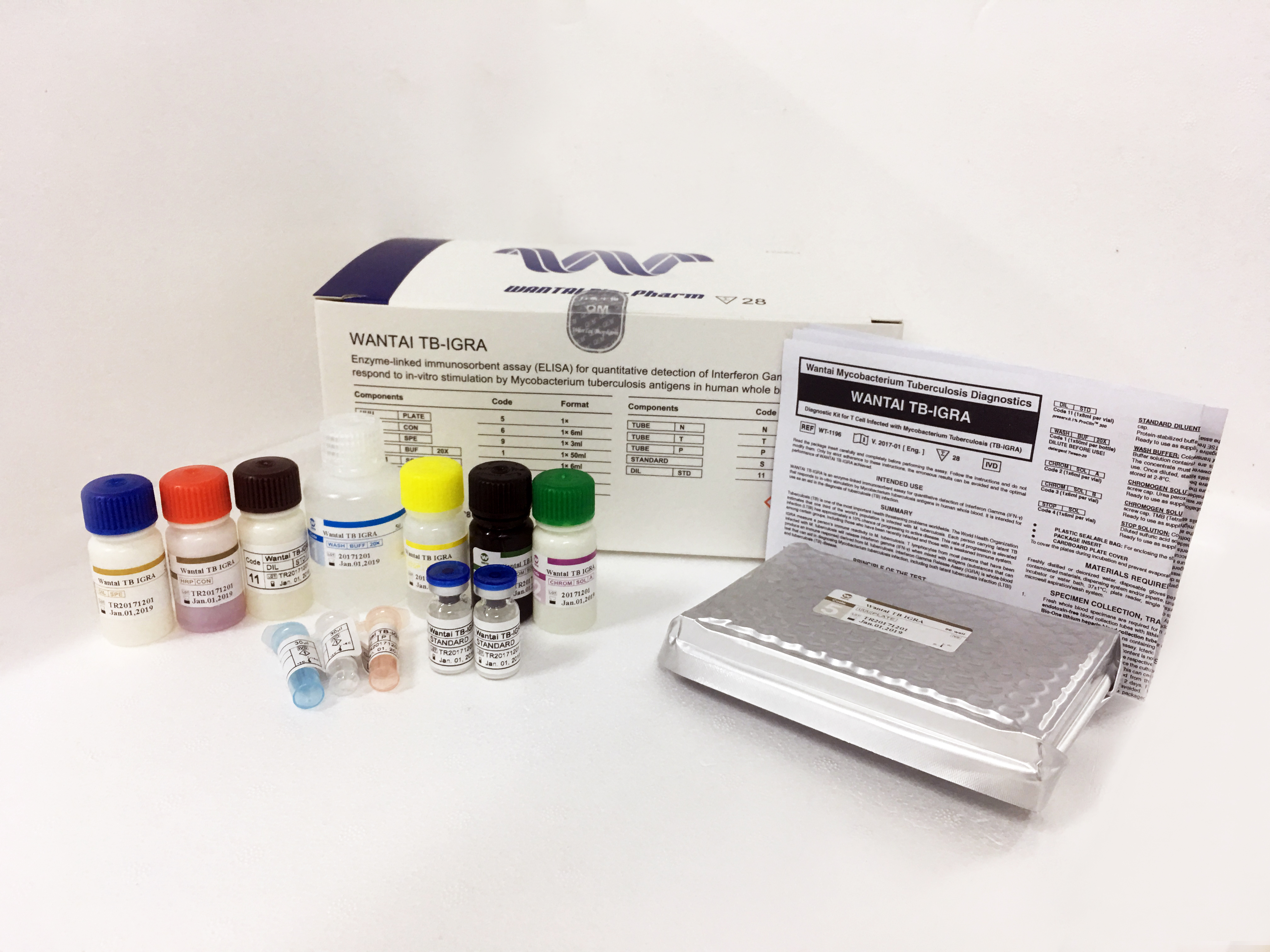
Wantai TB-IGRA Test Kits
Intended Use
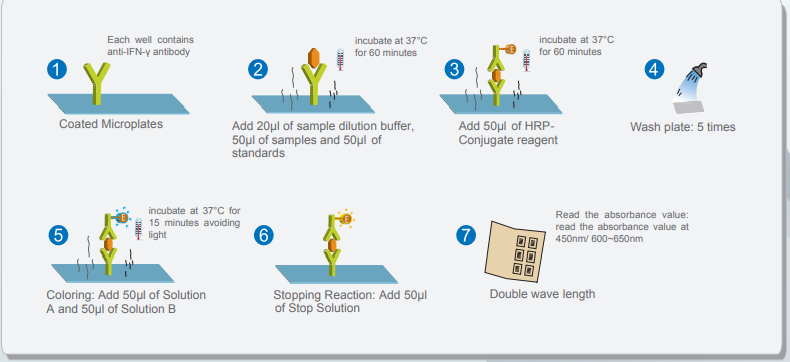
Wantai’s TB-IGRA ELISA is intended for use as an aid in the diagnosis of TB infection including both latent Tuberculosis infection and Tuberculosis disease. The test is a quantitative ELISA for detection of Interferon Gamma (IFN-γ) that responds to in-vitro stimulation by Mycobacterium Tuberculosis antigens in human blood samples.
Procedure
1. Sample Preparation
2. Testing
Specifications
Catalog No.: WT-1196
Number of tests : 28 tests per kit
Storage temperature: 2℃ ~ 8℃
Sensitivity: Comparable to both QFT-GIT (86.4% vs 83.2%) and T-Spot (87.7% vs 88.7%)
Specificity: Comparable to both GFT-GIT(85.9% vs 88.7%) and T-Spot (81.6% vs 91.9%)(1)
Recommendations
Wantai TB-IGRA ELISA is included in the Stop TB Partnership Manual on selection and use of interferon-gamma release assays (IGRAs) for testing for TB infection and in the WHO's policy statement on the use of alternative interferon-gamma release assays for the diagnosis of TB infection.
Resources
Wantai TB-IGRA ELISA - View Information Pack
Wantai TB-IGRA ELISA - Operation video
Reference:
1) New Commercial IGRAs for TB Infection • CID 2023:76
![]() Intended Use
Intended Use
Wantai’s TB-IGRA CLIA is intended for use as an aid in the diagnosis of TB infection including both latent Tuberculosis infection and Tuberculosis disease. The test is a quantitative chemiluminescence (CLIA) assay for detection of Interferon Gamma (IFN-γ) that responds to in-vitro stimulation by Mycobacterium Tuberculosis antigens in human blood samples.
Procedure
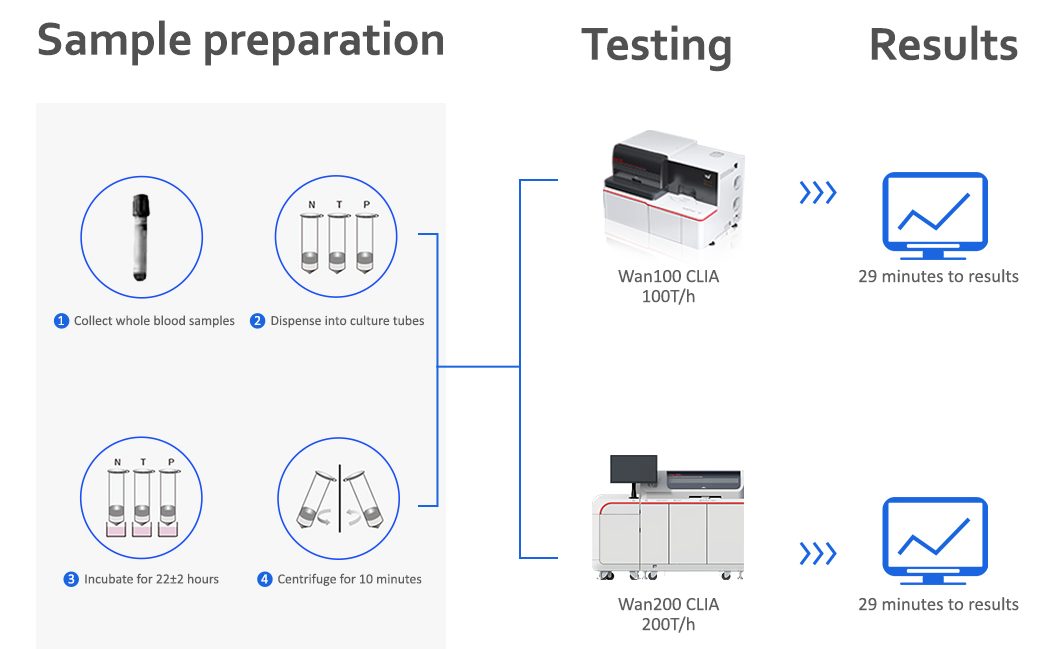
Specifications
Catalog No.: KR-TB-IGRA-01020/50
Number of tests : 20/50 tests per kit
Storage temperature: 2℃ ~ 8℃
Sensitivity: 98.72%
Specificity: 96.36%
Linear Range: 1pg/mL to 5000pg/mL
Applicable instruments
Wantai TB-IGRA CLIA is available on Wantai's Wan100 and Wan200+ chemiluminescence immunoassay analyzers.

Resources
Wantai TB-IGRA CLIA - View Information Pack

Intended Use
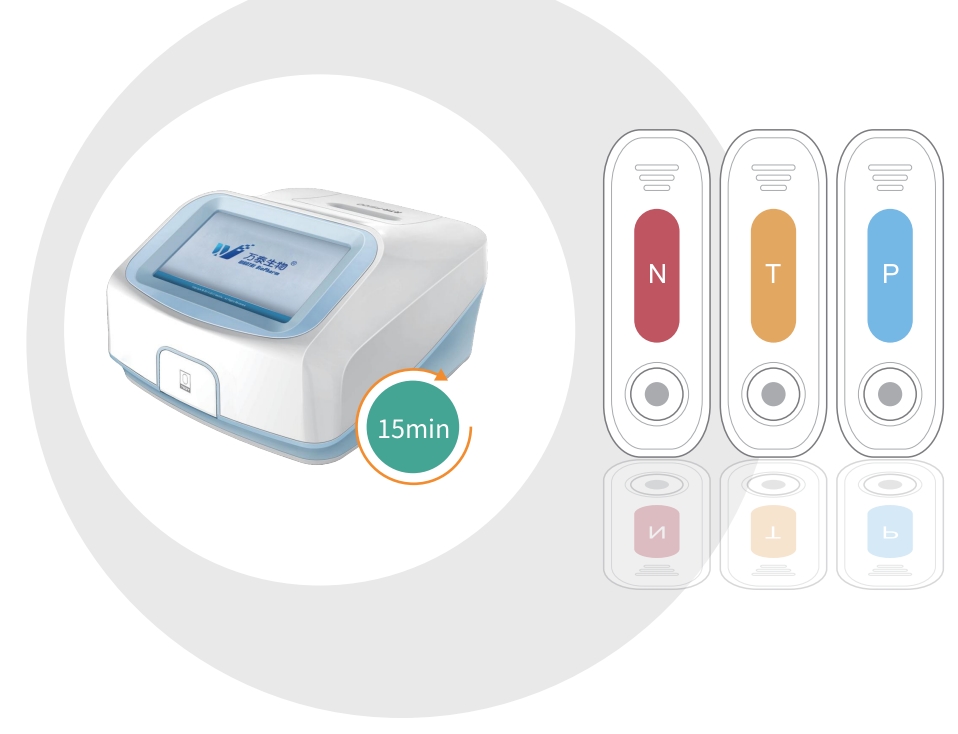
Wantai’s TB-IGRA FIA is intended for use as an aid in the diagnosis of TB infection including both latent Tuberculosis infection and Tuberculosis disease. Wantai’s TB-IGRA FIA is a fluorescence semi-quantitative test for detection of Interferon Gamma (IFN-γ) that responds to in-vitro stimulation by Mycobacterium Tuberculosis antigens in human blood samples.
Advantages: single sample testing with results within 15min, true POCT platform.
Procedure
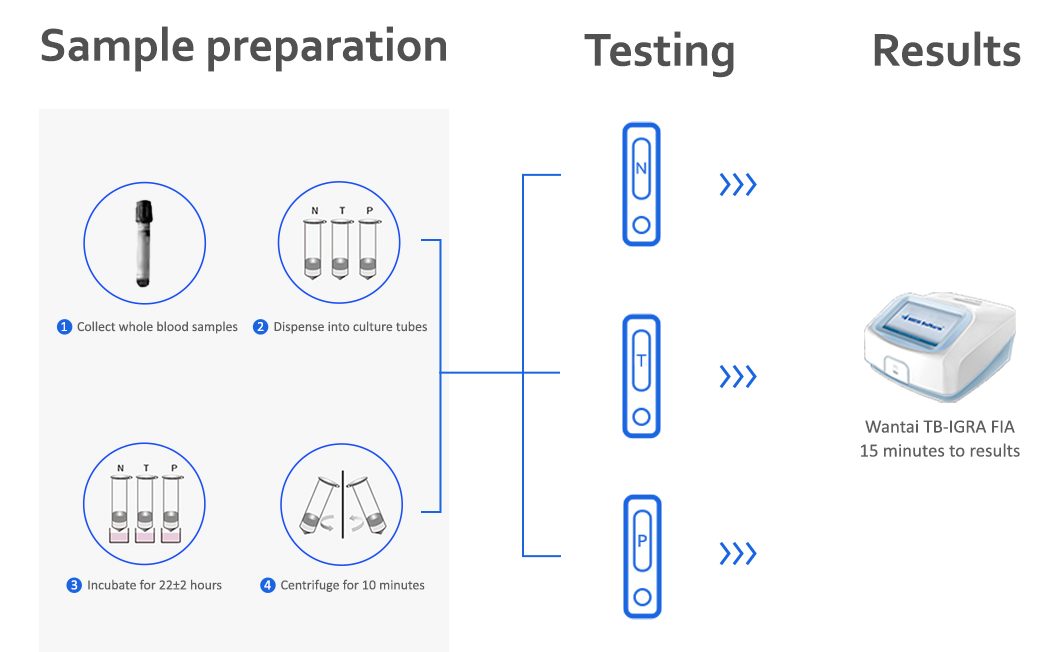
Specifications
Catalog No.: WJ-3110
Number of tests : 10 tests per kit
Storage temperature: 2℃ ~ 8℃
Sensitivity: 98.2%
Specificity: 97%
Applicable instrument: FS100
Resources
Wantai TB-IGRA FIA - View Information Pack