
20,000,000
Antigen Test Production Capacity per Month
Along with the spreading global pandemic of Novel Coronavirus, countries around the world have been struggling to diagnose and control this disease in time. Xiamen University and Wantai have jointly developed a series of innovative, highly sensitive and specific serological and molecular assays for testing of Covid-19. Together our tests offer total testing solution for Covid-19 diagnosis with results available in just 75-minutes.
Wantai SARS-CoV-2 Manual Diagnostic Kits:
Rapid Test for SARS-CoV-2 Antigen (colloidal gold)![]()
CE Annex - IV Self-Test CE2854 | Anterior Nasal Swabs and Saliva
CE Professional Use, EU Common List | Anterior Nasal Swabs
WANTAI SARS-COV-2 Antigen Rapid Test (colloidal gold) is a lateral flow test intended for qualitative detection of SARS-CoV-2 nucleocapsid antigen in anterior nasal and saliva specimens. Time to results: 15~20min.
Download Self-Test Operational Guide
View below training video (English).
This video is also available in French , Turkish , Danish , Finnish , Swedish, Spanish , German
Rapid Test for SARS-CoV-2 Antigen ( colloidal gold)
Australia - TGA Approved
Nasopharyngeal (NP)/oropharyngeal (OP) Swabs | Professional Use
WANTAI SARS-COV-2 Antigen Rapid Test (colloidal gold) is a lateral flow assay intended for qualitative detection of SARS-CoV-2 nucleocapsid antigen in nasopharyngeal (NP) and oropharyngeal (OP) swab specimens. Time to results: 15~20min.
View below training video.
Rapid Test for SARS-CoV-2 Antigen ( FIA ) ![]()
Nasopharyngeal (NP)/oropharyngeal (OP) swabs | Professional Use
Download Products Brochure , Download Procedure Diagram
WANTAI SARS-COV-2 Antigen Rapid Test is a lateral flow immunofluorescent assay (FIA) intended for qualitative detection of SARS-CoV-2 nucleocapsid antigen in nasopharyngeal (NP) and oropharyngeal (OP) swab specimens collected into viral transport media (VTM). Time to results: 15~20min.
View below training video.
SARS-CoV-2 Antigen ELISA Download Product Brochure ![]()
WANTAI SARS-COV-2 Ag ELISA is an enzyme-linked immunosorbent assay for qualitative detection of SARS-CoV-2 nucleocapsid antigen in nasopharyngeal (NP)/oropharyngeal (OP) swab, and serum or plasma specimens. It is intended for screening of patients suspected for infection with SARS-CoV-2 virus, and as an aid in the diagnosis of the coronavirus disease 2019 (COVID-19).
SARS-CoV-2 IGRA ELISA (Quantitative) - Download Brochure![]()
WANTAI SARS-CoV-2 IGRA is an enzyme-linked immunosorbent assay for quantitative detection of Interferon Gamma (IFN-γ) that responds to in-vitro stimulation by spike protein of SARS-CoV-2 in human whole blood. It is intended for use as an aid in the diagnosis of specific T cellular immune response of SARS-CoV-2 spike protein after vaccination or infection.
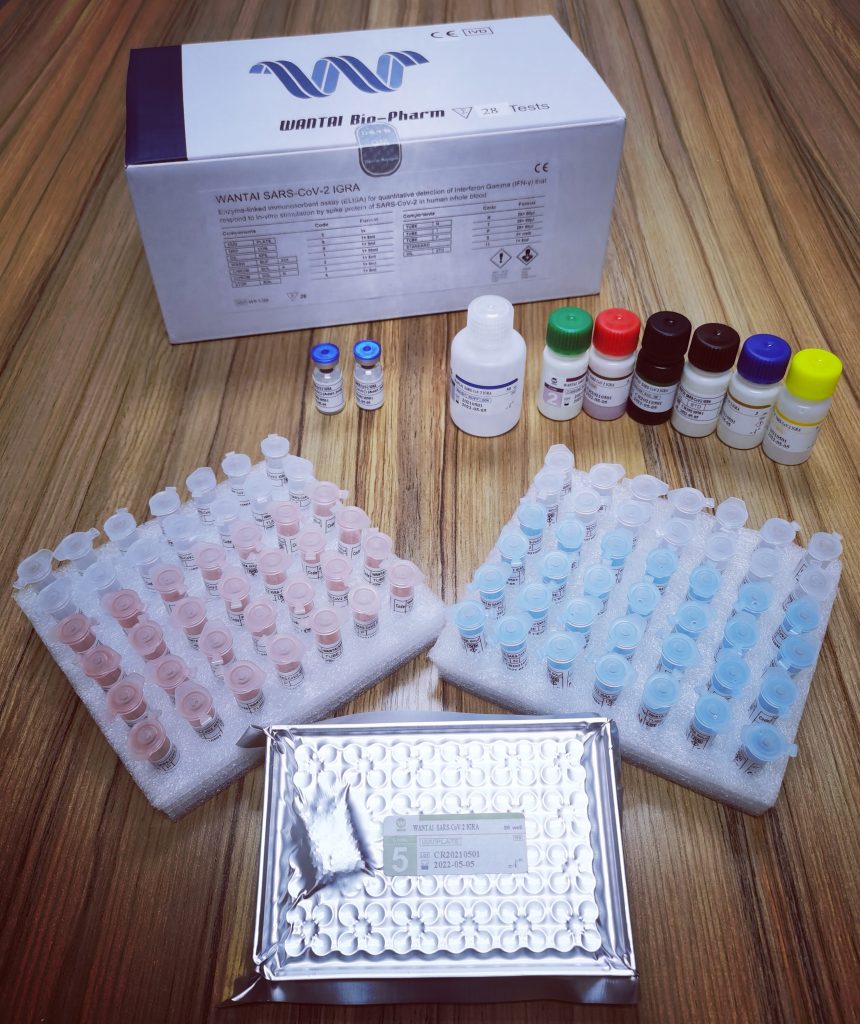
SARS-CoV-2 Neutralizing Antibodies ELISA (Quantitative) - Download Brochure![]()
WANTAI SARS-CoV-2 NAbs ELISA is an enzyme-linked immunosorbent assay for the quantitative detection of neutralizing antibodies to SARS-CoV-2 virus in human serum or plasma. The test is intended for use as an aid in identifying individuals with an adaptive immune response to SARS-CoV-2, indicating prior infection, or as an aid in individual vaccination management decisions.
SARS-CoV-2 IgG ELISA (Quantitative) - Download Brochure![]()
WANTAI SARS-CoV-2 IgG ELISA (Quantitative) is an Enzyme-Linked Immunosorbent Assay (ELISA) intended for quantitative detection of IgG-class antibodies to SARS-CoV-2 virus in human serum or plasma. The WANTAI SARS-CoV-2 IgG ELISA (Quantitative) is intended for use as an aid in identifying individuals with an adaptive immune response to SARS-CoV-2, indicating recent or prior infection, or as an aid in individual vaccination management decisions. The quantitative result obtained with this kit is as a reference for clinician only, cannot be used as the sole basis for further individual vaccination and treatment.
SARS-CoV-2 Total Ab ELISA - Download Brochure ![]()
WANTAI SARS-CoV-2 Ab ELISA detects total antibody as indicative of an immune response to SARS-CoV-2 in patients suspected of previous SARS-CoV-2 infection, or for the detection of seroconversion in patients following known recent SARS-CoV-2 infection. The test may also be used to aid in the diagnosis of acute or past SARS-CoV-2 infection in conjunction with other tests and clinical information. The prevalence of SARS-CoV-2 infection in the area where testing has occurred should be considered when interpreting positive test results. The test should not be used as the sole basis for diagnosis.
Performance evaluations (click on the links to open each study report)
The kit detects TOTAL ANTIBODIES (IgG, IgM and IgA) against S-RBD and it has been extensively evaluated and validated in Europe.
In the Netherlands, reporting by the Dutch Serology Taskforces shows sensitivity of the kit of 98.1% for samples collected > 14 days after onset of illness, the Dutch Serology Taskforce report can be downloaded from HERE. In other studies conducted in the Netherlands, Erasmus Medical Center showed sensitivity of 98% (100% >14days) and Sanquin Blood Bank calculated PPV of 99%, 88%, and 72% in areas with prevalence of 4-10%, 2-4%, and <2%. .
Statens Serum Institut in Denmark showed sensitivity of 71% (7~13days) ~ 100% (10days) . Also in Denmark, multicentre comparison study ranked Wantai SARS-CoV-2 Ab ELISA as the top serology assay among 15 commercially evaluated tests including automated immunoassays. The Medizinische Universität Wien in Austria calculated sensitivity of 92~100% , and in France, the French National Reference Center (CNR) evaluated the kit and concluded that it has sensitivity of 100% (7-13days), 95% (14-19days) and 98% (>20days) respectively. Over 98% agreement with automated immunoassays has been demonstrated during a study conducted by the University Hospital in Padova, Italy. In Belgium, AZ Delta Medical Laboratories and Ghent University concluded that Wantai SARS-COV-2 Ab ELISA is suitable for sensitive and specific screening of a SARS-CoV-2 infection from 10 days after symptom onset. ICMR/NIV in India evaluated the kit, the sensitivity was 98% and the specificity was 100%.
In the United States, validation study conducted by the National Cancer Institute (NCI) showed sensitivity of 96.7% (29/30) and specificity of 97.5% (78/80) of the test. Subsequently, WANTAI SARS-CoV-2 Ab ELISA received FDA Emergency Use Authorization on August.05,2020. Downloads: Letter of Authorization , HCP , Recipients , IFU , NCI Report.
FIND results are expected soon.

SARS-CoV-2 lgM ELISA - Download Brochure ![]()
Wantai SARS-CoV-2 lgM ELISA is an enzyme-linked immunosorbent assay for the qualitative detection of lgM-class antibodies to SARS-CoV-19 virus in human serum or plasma. It is intended for testing of patients suspected of recent infection with the SARS-CoV-2 virus. The test should not be used as the sole basis for diagnosis. The test is based on antibody-capture method.
Performance evaluations (click on the links to open each study report)
The Medizinische Universität Wien in Austria reported sensitivity of 92% (6 ~10days) to 100% after day 11 post disease onset, and 97% specificity of the kit. In the Netherlands, data collection and reporting by the Serology Taskforce, which is part of the Dutch National Testing Capacity Coordination Structure shows sensitivity of the kit of 91.9% for samples collected > 14 days after onset of illness. The Dutch Serology Taskforce report can be downloaded from HERE.
FIND results are expected soon.

Rapid Test for semi-quantitative detection of IgG antibody to SARS-CoV-2 Click to Download Brochure ![]()
! IMPORTANT : THIS PRODUCT IS INTENDED FOR PROFESSIONAL USE ONLY, NOT FOR SELF-TESTING OR TESTING AT HOME !
The WANTAI SARS-CoV-2 IgG Rapid Test (Semi-Quantitative) is a lateral flow assay for the semi-quantitative detection of IgG antibody against SARS-CoV-2 in human serum or plasma. The test is intended for use as an aid in detecting immune response level for individuals infected with SARS-CoV-2, indicating recent or prior infection, or individuals who have been vaccinated with COVID-19 vaccines. The quantitative results obtained with this kit are for clinical reference only, should not be used as the sole basis for vaccine immunization or treatment management.
Rapid Test for Total Antibody to SARS-CoV-2 Click to Download Brochure![]()
! IMPORTANT : THIS PRODUCT IS INTENDED FOR PROFESSIONAL USE ONLY, NOT FOR SELF-TESTING OR TESTING AT HOME !
WANTAI SARS-CoV-2 Ab Rapid Test is a 15minutes test that detects total antibody as indicative of an immune response to SARS-CoV-2 in patients suspected of previous SARS-CoV-2 infection, or for the detection of seroconversion in patients following known recent SARS-CoV-2 infection. The kit is based on double antigen "sandwich" method and it detects TOTAL ANTIBODIES against S-RBD. The test may also be used to aid in the diagnosis of acute or past SARS-CoV-2 infection in conjunction with other tests and clinical information. The prevalence of SARS-CoV-2 infection in the area where testing has occurred should be considered when interpreting positive test results. The test should not be used as the sole basis for diagnosis.
Performance evaluations (click on the links to open each study report) :
In a limited validations, the RIVM National Institute for Public Health in the Netherlands, and the Ministry of Health in the Czech Republic both reported sensitivity and specificity of 100%. In Austria, the Medizinische Universität Wien calculated sensitivity of 80% (6-10days) and 100% (>11days) respectively, and the University of Innsbruck reported sensitivity of 95.7% (100% >14days) and specificity of 100%. In France, the French National Reference Center (CNR) evaluated the kit and conclude that it has sensitivity of 89% (7-13days), 92% (14-19days) and 94% (>20days) respectively.
In the United States, validation study conducted by the National Cancer Institute (NCI) showed sensitivity of 100% (30/30) and specificity of 98.8% (79/80) of the test. Subsequently, WANTAI SARS-CoV-2 Ab Rapid Test received FDA Emergency Use Authorization on July.10,2020. Downloads: Letter of Authorization , HCP , Recipients , IFU , NCI Report.
FIND evaluation results are expected soon.
View our how to operate video.
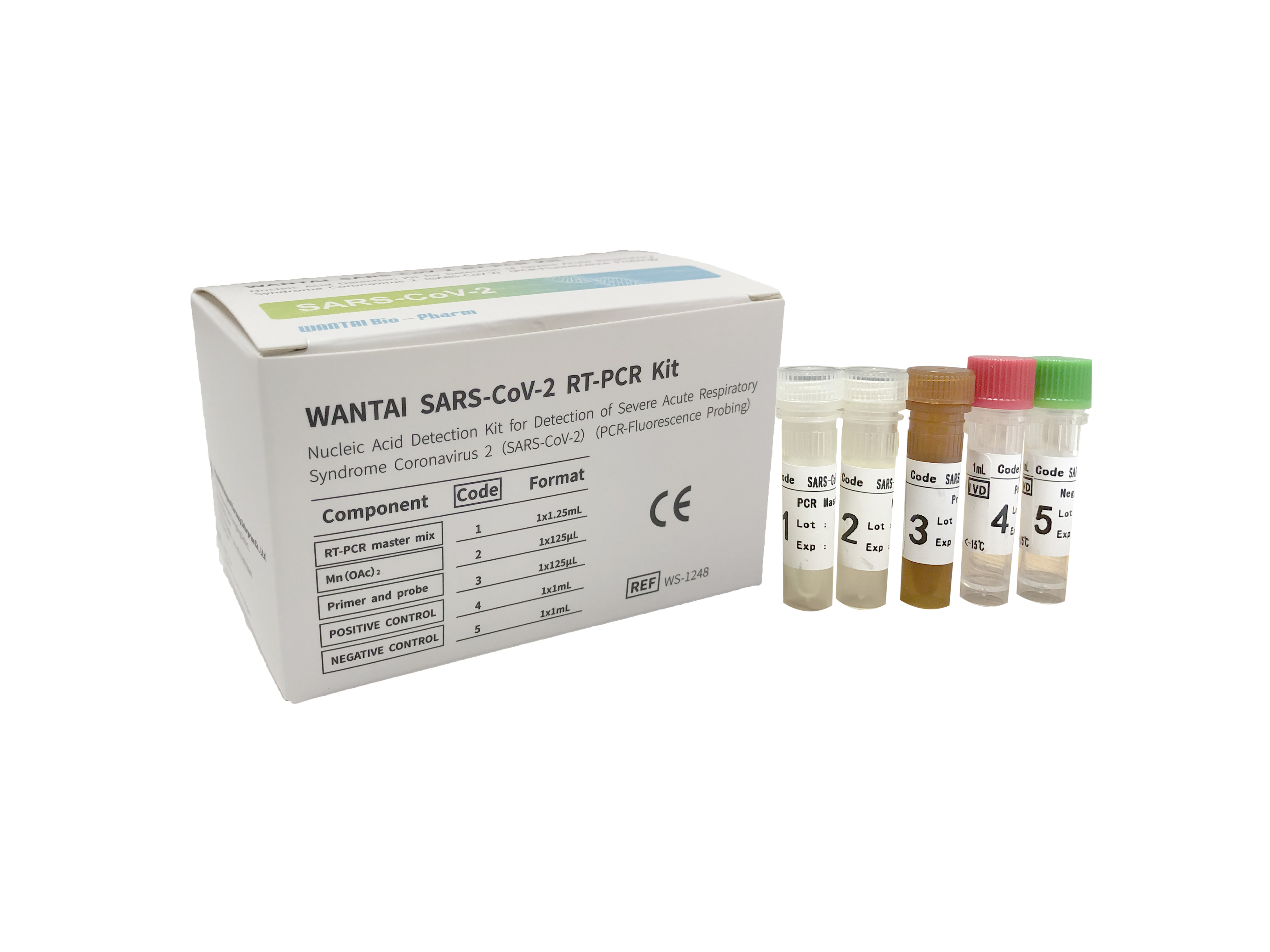
SARS-CoV-2 RT-PCR Download Brochure ![]()
Wantai SARS-CoV-2 RT-PCR Kit is a Nucleic Acid Detection kit for the detection of Severe Acute Respiratory Syndrome Coronavirus (PCR – Fluorescence Probing).
This kit is intended for detection of the Severe Acute Respiratory Syndrome Coronavirus 2 (SARS-CoV-2 ) RNA extracted from oropharyngeal swab, nasopharyngeal swab, sputum, endotracheal aspirate and bronchoalveolar lavage fluid samples of patients suspected for infection with COVID-19.
Wantai SARS-CoV-2 RT-PCR is a qualitative, real-time fluorescent PCR in which specific primers and fluorescent probes are designed to detect the highly conservative regions of the ORF1ab and N genes of the virus. This kit has integrated quality control (IC, human housekeeping gene) intended for monitoring of the test run.
LoD: 50 copies/ml
Ct cut-off: ≤40
Clinical sensitivity (50 positives): 100% (95%CI: 93 ~ 100)
Clinical specificity (100 negatives): 100%(95%CI: 96 ~ 100)
FIND evaluation results are available here.
WHO Emergency Use Listing (EUL) - Wantai SARS-CoV-2 RT-PCR was listed as eligible for WHO procurement on 14 August 2020. The kit also received FDA Emergency Use Authorization on September.09,2020. FDA downloads: Letter of Authorization , HCP , Recipients , IFU.
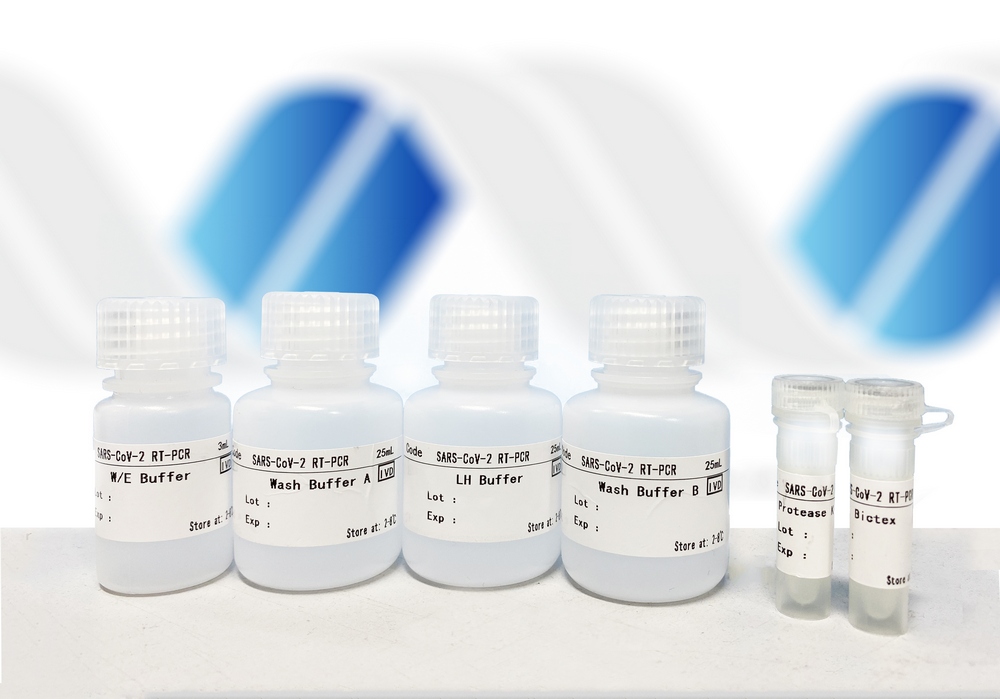
Nucleic Acid Extraction Kit ![]()
Nucleic Acid Extraction Kit for isolating viral DNA and RNA using superparamagnetic beads, the extracted nucleic acid is applicable for molecular detection such as PCR and real time-PCR. High purity viral nucleic acid can be extracted from human nasopharyngeal swabs, sputum, broncho lavage and alveolar lavage fluid samples.
- Purity (A260/A280) : DNA 1.8 ~ 1.9 ; RNA 1.9 ~ 2.0
- Extraction Efficiency : >95%
Recommended Extraction Instrument : NEXOR 32 ![]()

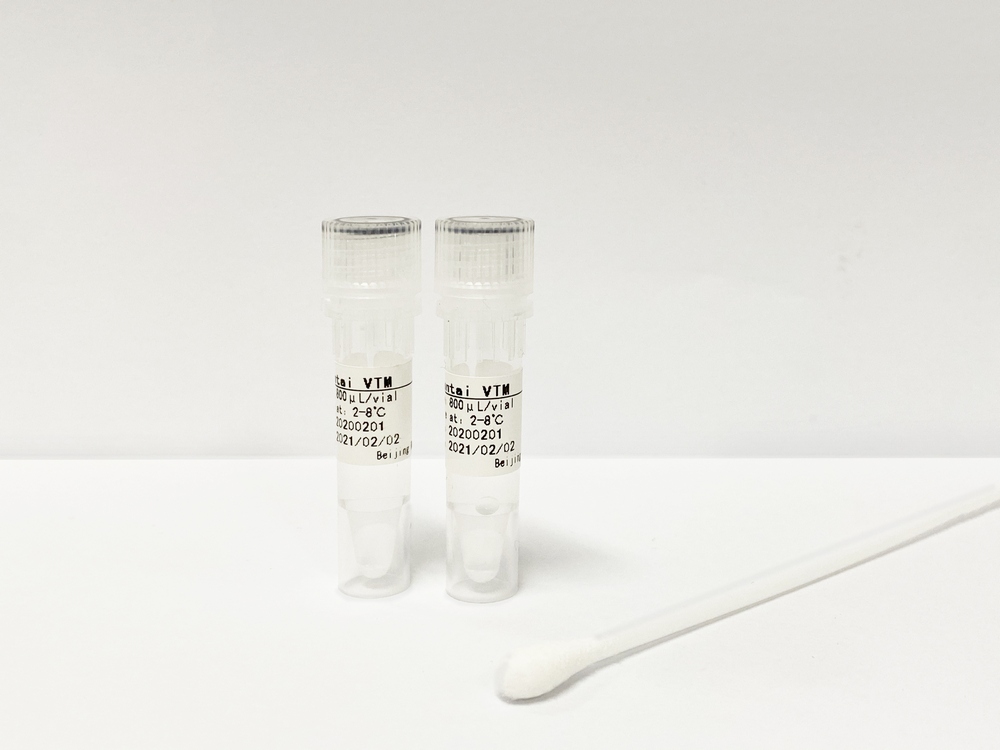
Viral Transport Media (VTM) ![]()
Viral Transport Media - transportation and storage tube with virus lysis buffer.
Clinical specimens collected with nasopharyngeal and oropharyngeal swabs can be dissolved into this product for later testing or storage. This product contains appropriate concentrations of guanidine isothiocyanate and other components for cellular lysis to effectively suppress the activity of DNase/RNase in specimens, which contributes to the stable storage of the specimen’s nucleic acid without affecting its extraction/purification and amplification tests.
Authorized Distributors
Greece: New Diagnostic Dimension Ltd
Telephone: +30 210 7754303

Nordic countries: Nordic Biosite
Telephone: +46(0)8-54443340

Austria & Switzerland: SZABO-SCANDIC
Telephone: +43-1-489 3961-0

Benelux: Sanbio B.V.
Telephone: +31 413 251115

Germany: Sanbio B.V.
Sanbio B.V.
Telephone: +31 413 251115

Germany: Borind International GmbH
Telephone:+49(0)2065 7915313
www.biovigo.de

Germany: Human Diagnostica
Telephone: +49 6122 9988-0
info@human.de

Iberia: Quilaban
Telephone: +351 21 923 63 50

France: Eurobio Scientific
Telephone: +33 1 69 79 18 26

Italy: D.B.A. ITALIA s.r.l.
Telephone: 02 2692 2300

Balkans, Africa and South America (non exclusive): Human Diagnostica
Telephone: +49 6122 9988-0
info@human.de

Czech & Slovak Republics
Kommand Team s.r.o.
* Antibody Rapid Test
* Nasal and Saliva Swabs Antigen Test
Telephone: +420776606860
AKESO holding a.s.
Antigen Rapid Test OP/NP swabs
www.akesoholding.cz

Testline (ELISA)
Telephone: +420 549 121 239

Russia & CIS: Cosmopharm LLC
Telephone: +7 (495) 644 00 31

UK & Ireland: Cambridge Bioscience
Telephone: +44( 0) 1223 316 855

Australia, New Zealand and the Caribbean: Suretest
Telephone: +61449994615

USA: Dynex Technologies
Telephone: +1 703.631.7800
customerservice@dynextechnologies.com

Mexico, Venezuela, Colombia : Laboratorios DAI
Telephone: +57 1 2122744 (Colombia, Venezuela)
Telephone: +52 (55) 2624-2500 (Mexico)

Ecuaodor : Vibag C.A.
Telephone: +593 4288407 Ext. 113

Chile :
Vytrous
Telephone: +56 9 65183943
www.vytrous.com

Biocant
Telephone: +56 226830900
www.biocant.cl

The Philippines: Caffrey Trading Co.
Telephone: 0956-188-8873 / 0951-336-9656
Thailand : Hippo Marketing
Telephone: +66 66-164-9199

