HIV
The human immunodeficiency viruses type 1 and type 2 are the etiological agents of the acquired immunodeficiency syndrome (AIDS). HIV has been isolated from patients with AIDS, AIDS related complex (ARC) and from healthy individuals at high risk for AIDS. Serological evidence of infection with HIV may be obtained by testing for presence of HIV antigens or antibodies in serum of individuals suspected for HIV infection. Antigens can generally be detected during both acute phase and the symptomatic phase of AIDS only. The Antibodies to HIV-1 and/or HIV-2 can be detected throughout virtually the whole infection period, starting at, or shortly after the acute phase and lasting till the end stage of AIDS. Apart from sexual transmission, the principal route of infection with HIV is blood transfusion. HIV can present both in cellular and cell-free fractions of human blood. Therefore, all donations of blood or plasma should be tested due to the risk of HIV transmission through contaminated blood.
HIV Diagnostic Test Kits
HIV Ab ELISA - CE View Information Pack
Wantai AiDTM anti-HIV 1+2 ELISA is an enzyme-linked immunosorbent assay (ELISA) intended for qualitative detection of antibodies to Human Immunodeficiency Viruses (HIV) type 1 (group M - O) or type 2 in human serum or plasma specimens. The assay can be utilized for screening of blood donors and/or as an aid in the diagnosis of clinical conditions related to infection with HIV-1 and /or HIV-2 - the etiological agents of the acquired immunodeficiency syndrome (AIDS).
HIV Ag/Ab ELISA - CE View Information Pack
Wantai AiDTM HIV 1+2 Ag/Ab ELISA Plus is an enzyme-linked immunosorbent assay (ELISA) intended for qualitative detection of antigens and/or antibodies to Human Immunodeficiency Viruses (HIV) type 1 (group M - O) and/or type 2 in human serum or plasma specimens. The method is also known as 4th generation ELISA for HIV detection. The kit is intended for screening of blood donors and as an aid in the diagnosis of clinical conditions related to infection with HIV-1 and/or HIV-2 - the etiological agents of the acquired immunodeficiency syndrome (AIDS).
HIV 1+2 Rapid Test - CE View Information Pack
The HIV 1+2 Rapid Test is a single-use rapid device for qualitative detection of antibodies to Human Immunodeficiency Viruses (HIV) in blood, serum and plasma specimens. The test is intended for use in health facilities by trained staff as an aid for the diagnosis of clinical conditions related to infection with HIV-1 and/or HIV-2 – the etiological agents of the acquired immunodeficiency syndrome (AIDS). It provides a clear result within 10-30 minutes.
Rest-of-World regulatory version of this product was accepted for the WHO list of prequalified in vitro diagnostics and was listed on 15 February 2016. The PUBLIC REPORT can be downloaded HERE. View Information Pack
HIV Dot ELISA (saliva samples) - View Information Pack
This highly sensitive test can effectively and rapidly detect antibodies to HIV-1 and HIV-2 in oral mucosal transudate fluid. It provides results within 30 minutes.
HIV SELF-TEST BY URINE - Watch video View Information Pack
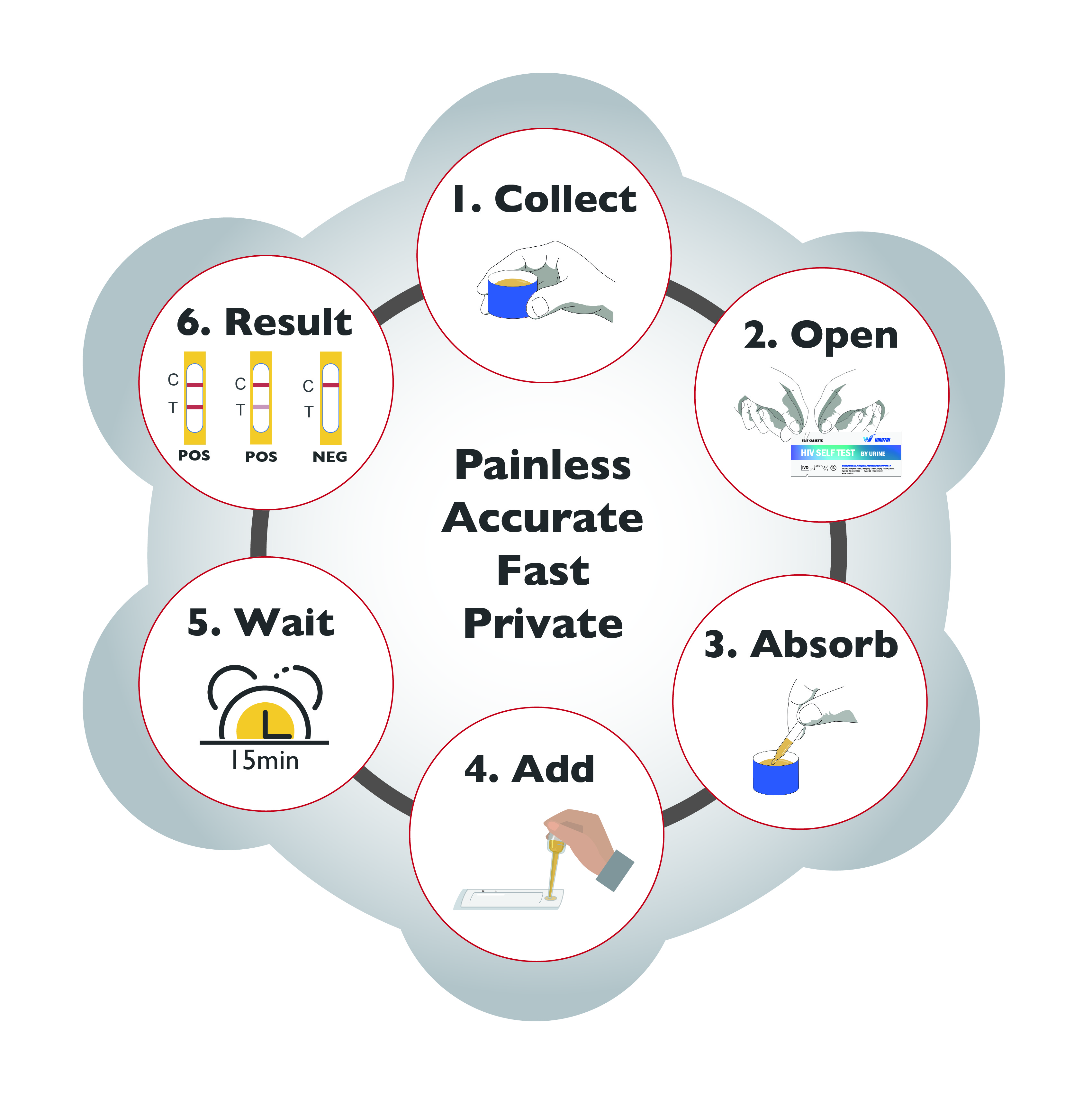
Countries want to rapidly increase HIV testing services especially for people with low access and at at higher risk. One approach to do this is HIV self-testing, where a person collects his or her own specimen and then performs the test and interprets the result by himself or herself.
Wantai HIV URINE Self-test is a rapid HIV test intended for use by untrained individual to test himself or herself at home.
* Easier to collect specimen than blood or oral fluid
* Painless specimen collecting procedure
* Easy to use - only 6 procedure steps
* Fast - time to results is only 15minutes
* Easy results interpretation, visual interpretation
* Private - no need for supervision by lab specialist
* Compact - all materials in one box. No liquid buffer
* Accurate - over 99% accuracy to the lab test
* Safer disposal after use
* 18 months shelf-life
* Room temperature storage

HIV 1+2 Confirmation Test - View Information Pack
Beijing Wantai confirmation tests are intended for use as supplemental tests for human serum or plasma specimens found to be repeatedly active in HCV and HIV antibody screening procedures. Tests used for the diagnosis of HIV and HCV infection require a high degree of both sensitivity and specificity, which is achieved using an algorithm combining two tests. Once antibodies have been detected by an initial ELISA test, a second supplemental test using the immunoblot procedure is applied and confirms or excludes the results of the initial ELISA test.
HTLV
The Human T-cell Lymphotropic viruses (HTLV 1 and HTLV 2) are a member of the family of Retroviridae, consisting of enveloped double stranded RNA viruses. Both types of HTLV are transmitted transplacentally, parenterally, by sexual contact and by infected blood. While routes of transmission and latency periods are similar to those of HIV, the two are genetically non-related. HTLV 1 commonly infects CD4+ lymphocytes, but infection of CD8+ T lymphocytes can also occur. HTLV 2 can infect all type of lymphocytes as well as the macrophages. The diseases associated with HTLV infections are usually classified as malignant or non-malignant clinical presentations.
HTLV 1 is most commonly found in southern Japan, the Caribbean and the US, but also occurs in other parts of the world. While endemic in some North American Indian tribes, HTLV 2 is detected mostly in intravenous drug users and their sexual partners.
HTLV Diagnostic Test Kits
HTLV Antibody Diagnostic ELISA Kit - View Information Pack
Wantai HTLV 1+2 ELISA is an antigen “sandwich” used for qualitative detection of antibodies to HTLV type 1 and/or 2 in human serum or plasma. It is intended for screening of blood donors and as an aid for the diagnosis of clinical conditions related to infection with HTLV-1 and/or HTLV-2. The diagnostic procedure takes 90 minutes. Both sensitivity and specificity are very high - sensitivity is 100%, while specificity is 99.97%.
